
A July tornado that tore across the Rocky Mount, N.C., region left severe damage including to a Pfizer facility. Amid subsequent supply chain disruption, the manufacturer alerted hospitals to a list of 12 drugs available only through emergency orders “due to their high medical need,” effective “immediately and until further notice.” The disruption further prompted action from risk management expert Clifford Rossi and students in the Master of Quantitative Finance (MQF) program at the University of Maryland’s Robert H. Smith School of Business.
They produced a risk assessment database covering 6,000-plus manufacturing — pharmaceutical and other — facilities registered with the Food and Drug Administration (FDA). Their findings show tornadoes as the third biggest exposure for these facilities, with riverine flooding another significant risk — more than hurricanes, coastal flooding and wildfires.
“Extreme weather events and increased concerns over climate change further pressure already-strained drug supply chains and pose significant risk not only to the manufacturers, but also to insurers and end-users such as patients and consumers,” says Rossi, professor of the practice, executive-in-residence and director of the Smith Enterprise Risk Consortium. “Having the ability to know where potential hazard risk exists can be of enormous value to drug manufacturers in deciding where to locate plants as well as to harden them against these hazards.”
Rossi, with Smith MQF students Matthew Rumrill and Harini Mantripragada, analyzed the domestic drug manufacturing sites via the FDA's Drug Establishments Registration Site database in conjunction with data from FEMA's National Risk Index. The latter includes risk ratings for 18 different natural hazards and assigns an overall risk rating for each census tract and county in the United States.
Some notable takeaways, Rossi says, are:
- the natural hazards with the largest number of sites in high-risk areas, in order, are ‘lightning,’ ‘winter weather,’ ‘tornado,’ ‘strong wind’ and ‘ice storm;’
- about 42 percent of the total 6,024 FDA-registered manufacturing facilities including the pharma facilities (with operations including packaging, labeling and analysis) are in high-risk areas for tornadoes;
- by contrast, hurricane risk, is well down the list of the 18 hazards affecting the facilities, with just 11 percent of all sites in high-risk hurricane areas;
- riverine and coastal flooding came in at 31 percent and 6 percent, respectively, in terms of the percentage of the manufacturing facilities in high-risk flooding areas;
- nearly 17 percent of the facilities are in high-risk wildfire areas; and
- states with the highest-risk exposure to natural hazards are California, Florida and Texas. In California, about 95% of pharma plants are located in relatively-to-very high hazard areas across all hazard types.
Rossi, Rumrill and Mantripragada overlaid the FDA’s site-location information with the FEMA data to produce the following map and tables at the county and state level:
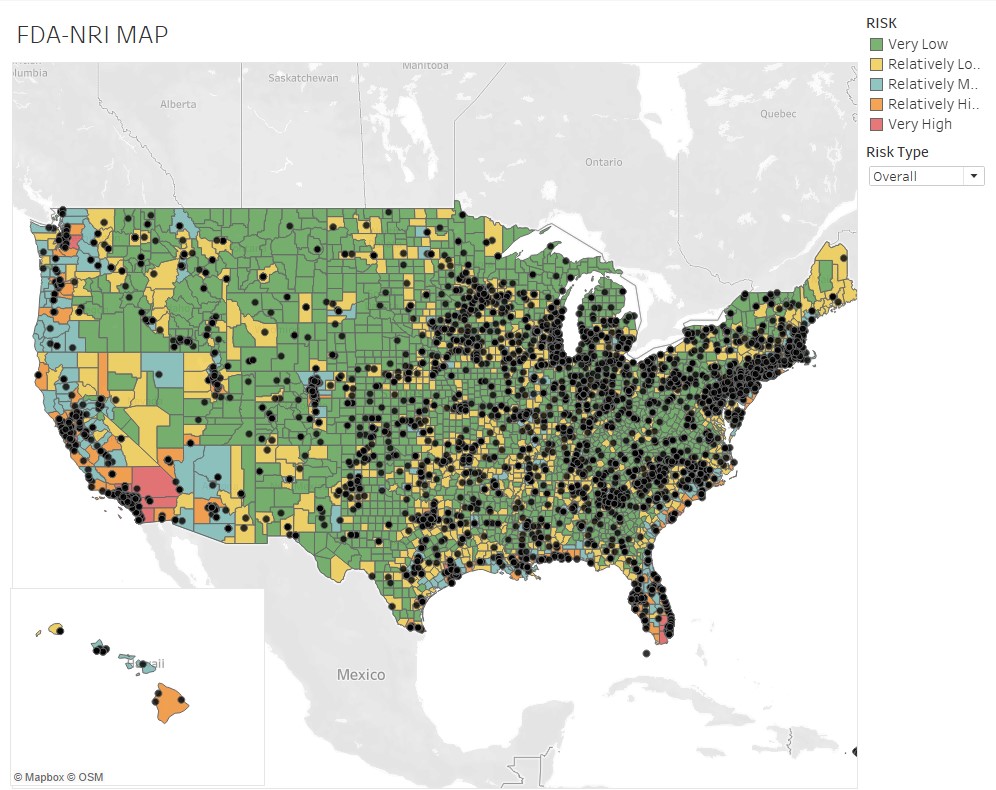
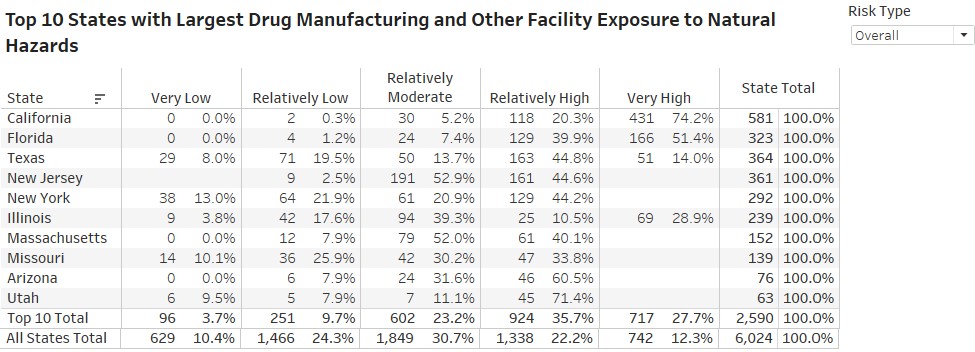
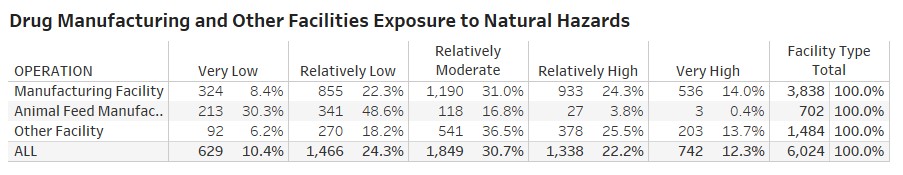
“The assessment’s implications are critical to maintaining a continuous supply of drugs across the U.S.,” Rossi says. “The work also illustrates Smith students — in this case Matt and Harini — producing analysis that’s real-time and impactful to markets.”
Read more about the Smith Enterprise Risk Consortium and Smith’s Business Master’s Programs.
Media Contact
Greg Muraski
Media Relations Manager
301-405-5283
301-892-0973 Mobile
gmuraski@umd.edu
Get Smith Brain Trust Delivered To Your Inbox Every Week
Business moves fast in the 21st century. Stay one step ahead with bite-sized business insights from the Smith School's world-class faculty.